ORIL has identified a family of plant-based compounds belonging to the steroid saponin family, determined their unique biological mode of action, demonstrated proof-of-principle in anti-cancer activity, and conducted preliminary preclinical and safety testing. Due to the vagary of natural supply of these compounds, ORIL has developed and patented a unique and highly specific method of synthesis for the lead candidates, scaled-up under GMP, together with the design and synthesis of novel structural analogues.
ORIL plans to advance its compounds along a planned development path to Phase I and II human clinical testing.
Preclinical Program
Drug Delivery Systems
The fundamental purpose of the drug delivery system (DDS or the drug formulation, i.e. its dosage form) is to provide a safe and effective vehicle for the drug to reach its intended target in the body (e.g. the tumour site). Speaking in broad terms, drugs can act systemically, when the drug reaches the body as a whole, including the tumour or locally, where the anti-cancer drug is directed into the part of the body where the cancer is located.
Systemic delivery includes parenteral and enteral administration. Examples of parenteral administration are intravenous (into a vein), intramuscular (into a muscle) and subcutaneous (under the skin). Examples of enteral administration include oral and rectal where the drug is absorbed from the gastrointestinal tract into the bloodstream.
A typical example of local administration is topical treatment, which commonly refers to application of the drug formulation to the skin but can also apply to other membrane surfaces such the rectal, vaginal, pulmonary, otic, oral and nasal cavities. An extension of topical administration is the transdermal DDS where the drug formulation is applied onto the skin surface but is absorbed through the skin into the bloodstream (e.g. patches for hormone or pain relief therapy).
Which DDS is chosen for a given drug largely depends on a number factors such as the drug’s physicochemical characteristics such as solubility, its pharmacokinetics, or the way (and rate) the drug is absorbed into the bloodstream, how it travels through the body to the tumour, how it is metabolised and eliminated, the type of tumour being treated and each patient’s health condition. Ultimately, the route of drug administration depends on this complex interplay between the properties of the drug itself, the disease and the patient. It is not surprising that the oral dosage form is usually the most popular with patients and clinicians alike due to convenience and ease of compliance; however this is not always technically possible for many drugs.
Ideally, drugs should be able to be formulated in different ways, to cater for a number of treatment conditions and circumstances but this is seldom the case. Unfortunately, the properties of many cancer drugs make them only suitable to be administered via one route of administration, limiting their application for a number of cancers and/or individual patients.
Importantly, the characteristics and mechanism of action of compounds of the ORIL family enable multiple formulation types. ORIL scientists are working on a number of DDS strategies that exploit the mechanism of action and physical characteristics of ORIL compounds. These different strategies will expand the range of applications of ORIL technology to a wider range of cancers and ultimately reaching more patients.
These different DDS strategies and example of applications are summarized in the figure below.
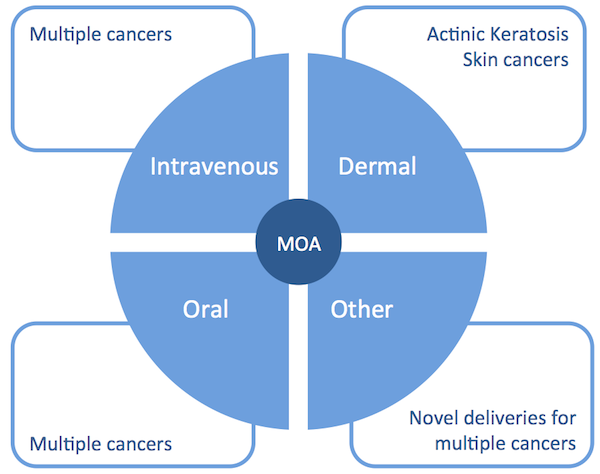
Clinical Program
The development strategy is designed to enable fast track of ORIL007 depending on the clinical outcomes from multiple Phase IB studies. Assuming positive outcomes this would (in consultation with FDA) enable pivotal Phase II study and orphan drug status. A summary is provided in the flow diagram below.
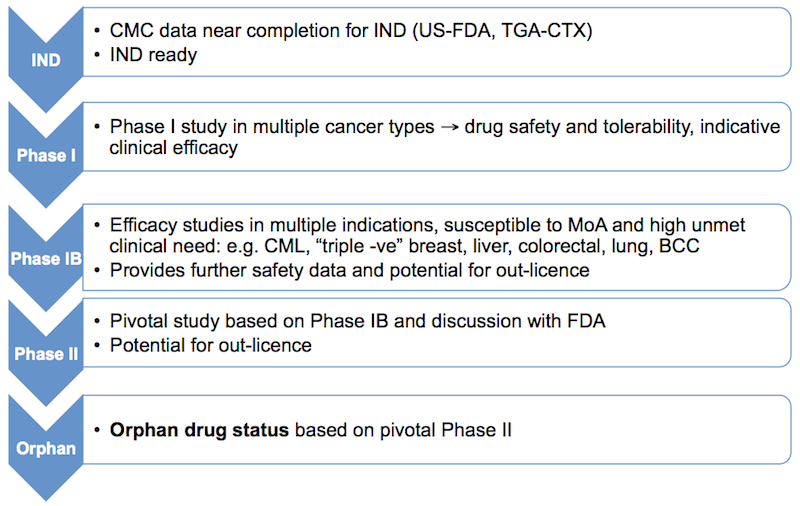
In parallel to the clinical and development program are ongoing studies that will underpin the technology and support the patent applications, thereby protecting ORIL’s valuable intellectual property.
Registered Office
Oncology Research International Limited
Level 5, 45 St George Terrace,
Perth WA 6000 Australia